Mauritius is signatory to the International health regulations (IHR 2005) that require a number of set core capacities to be fully functional by June 2016. Although, Mauritius has been making steady progress in several areas, there were some core capacities that need to be strengthened. The Joint External Evaluation (JEE evaluation) was conducted based on the WHO framework in October-November 2018. While the overall JEE score of the Core Capacities is 143 (60%) for Mauritius, this must be sustained and further advanced, by fully leveraging the IHR (2005) to strengthen their core capacities which consist of 19 technical areas, for responding effectively to both known and unknown public health threats in the future.
Towards this, and based on the findings of the mission and the recommended priority actions for each of the 19 technical areas, one of the five overarching thematic areas which have emerged is the strengthening of the data collection and surveillance systems, across human, animal and environmental health, as well as the public and private sectors, with integration and interoperability as the core underlying principles. This required strong and high-level commitment, as reporting of data were mostly paper-based and data collection was carried out on spreadsheets.
In this context, the Government of Mauritius through the Ministry of Health and Wellness prioritized the digitalisation of the health system and Dr the Hon Kailesh Kumar Singh Jagutpal, Minister of Health and Wellness, provided strong leadership and commitment towards prioritising the establishment of a strong unified and integrated web-based system which is critical in improving health sector policy formulation and programme implementation based on evidence-based decision making and ultimately positively impacting health outcomes in Mauritius. The aim is to strengthen the management of data collection, collation, analysis, interpretation and dissemination through its Health Management Information System (HMIS).
To this effect and following recommendations of the Mauritius Health Sector Strategic Plan 2020-2024, the Ministry of Health and Wellness set up the Mauritius National Core Team for District Health Information Software (DHIS2), which is led by the focal person, Dr Vijayesing Dinassing, with ongoing support and collaboration from World Health Organization (WHO), GAVI, Global Fund, HISP UiO and HISP Uganda. The Ministry of Information Technology, Communication and Innovation, provided a government administered server and DHIS2 was installed and customized as the open source software for routine reporting of health and health related data through an interoperable, interconnected, real-time electronic reporting system.
Following the implementation of DHIS2 for aggregate data collection for the Integrated Disease Surveillance and Response guidelines and the Expanded Programme of Immunization, the Ministry of Health and Wellness operationalized the National COVID-19 Vaccination Plan which proposed that individual data should be recorded using the DHIS2 COVID-19 Vaccine Delivery Toolkit package to support data administration. The National Pharmacovigilance Committee for Mauritius under the chairmanship of Dr Roupesh Jaggeshar, endorsed DHIS2 to record tracker data for adverse events following COVID-19 vaccinations. The DHIS2 AEFI Tracker Package was identified and the dashboard was customized using a set of indicators and an event report that was generated regularly, to be shared with stakeholders, once approval was obtained from the Ministry. The AEFI Tracker Package was then deployed in April 2021 after training of end-users and data administrators were completed from the regional to the central level. The Ministry was committed in providing the necessary infrastructures and internet facilities for the implementation of the AEFI surveillance package.
Mauritius, as a member WHO Programme for International Drug Monitoring (PIDM), is committed to the sharing of COVID 19 vaccines Individual Case Safety Reports (ICSRs) with the global database. In order to ensure timely reporting and submission to WHO VigiBase, interoperability was necessary between state members and IPDM, the HISP UiO team in collaboration with WHO and the VigiFlow team developed a working prototype for integrating VigiFlow and DHIS2 AEFI data. A key prominent features of this integration with VigiFlow is to facilitate the smooth dataflow to the global database (VigiBase) without duplication of efforts at the National Pharmacovigilance Level.
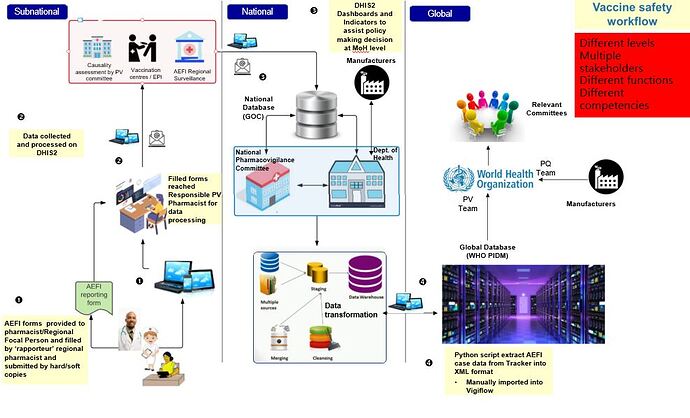
The diagram below illustrate the flow process of AEFI reporting from regional health facilities in Mauritius through the National Pharmacovigilance Committee at the central level to finally be uploaded in Global Database for the WHO PIDM. As demonstrated, AEFI forms are collected as hard copies by regional pharmacovigilance pharmacists which are then processed through DHIS2 and causality assessment is conducted by a panel of health experts both at national and sub-national level. The data collected on DHIS2 are stored on the National database and is administered by the Government online server of the Ministry of Information Technology, Communication and Innovation. The DHIS2 dashboard is updated in real time and provide indicators and analysis to assist decision makers in making informed decisions at central/national level. AEFI reporting to the Vigibase is then carried out through extraction of AEFI tracker data into XML format and imported into Vigiflow.
Mauritius was chosen as the first country to test this solution and once approval was obtained from the Ministry of Health and Wellness through the National Pharmacovigilance Committee, Mauritius, testing and validation were carried out, following which the Uppsala Monitoring Centre WHO Collaborating Centre for International Drug Monitoring and HISP UiO confirmed successful transfer of AEFI data from DHIS2 to VigiFlow in December 2021.
In its current prototype form, the integration between DHIS2 and VigiFlow is carried out using a Python script that extract AEFI case data from Tracker into XML format, which is then manually imported into VigiFlow and sent to VigiBase. This method has functioned successfully as a proof of concept. The next steps are to determine what steps of the extraction and import process can (or should) be automated, which depends both on technical factors and workflow considerations related to data review and approval.
Despite the achievements of implementing DHIS2 for AEFI surveillance from regional to the central level, further decentralisation of the system to the district and at primary health care level is required and thus, additional training, human resources and IT infrastructures are required. Additionally, the Republic of Mauritius also consist of Rodrigues and Agalega islands, where accessibility by the health facilities to the DHIS2 Tracker platform is currently limited. Therefore, there is a need to provide additional end-user training to healthcare staffs and database administrators who are from these remote islands, to ensure timely submission and report of AEFI cases.
To date, DHIS2 has been implemented for aggregate and tracker collection of data for the Expanded Programme of Immunization and has been fully operational for AEFI reporting, COVID-19 Vaccine Delivery, Hepatitis, HIV/AIDs and TB case surveillance within the Mauritius HMIS platform. Moving forward, we are hoping that the HISP network is strengthened for the Indian Ocean region to sustain DHIS2 implementation in the region as this will be critical for cross-border collaboration between the Indian Ocean islands in the context of disease surveillance and supporting monitoring and evaluation exercises for other health programmes in the Small Island Developing States (SIDS).